By David Calvert, June 2024
In our last post in this “Design for Formulation” series we discussed what should be done before you go into the laboratory, highlighting technical briefs and sources of previous knowledge. We ended by saying that a preliminary risk assessment should be carried out and here we briefly give an example.
Let’s take a look at a simple gel-based herbicide for consumer use. It has four components, the active ingredient, an adjuvant, a gelling agent (rheology modifier) and water. For the purposes of this example, the customer promise includes the statement “Will work in one application and 100% of the gel is applied to the weed.”.
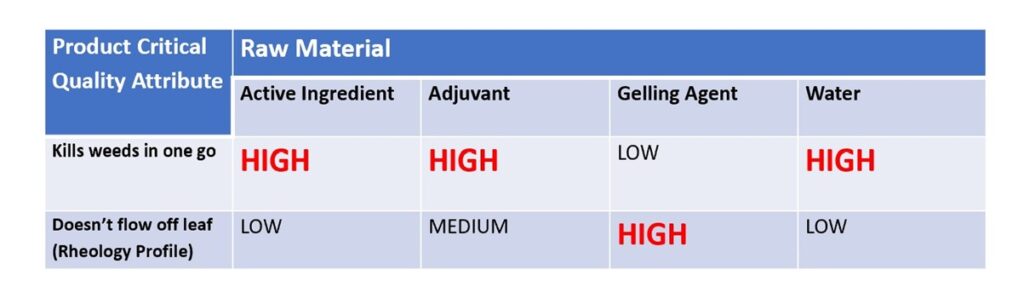
From this we established that two of the critical quality attributes were the amount and quality of active applied in one dose and that all of the applied product must stay on the weed. An initial risk assessment would establish the role each of the four ingredients would play in delivering the critical quality attribute. The effect/risk would be simply graded “high”, “medium” or “low”.
For the critical quality attribute “Will work in one go”, we assessed that the active ingredient, adjuvant and water were all “high”, whereas the gelling agent would be “low”. What this means is that if something was wrong with the active ingredient or adjuvant, then the “risk” of not delivering on the customer promise was high. The water was deemed as “high” as it is known that the hardness of water can sometimes impact negatively on performance of some actives in herbicides.
For the critical quality attribute “Doesn’t flow off leaf” then we assessed that the gelling agent is deemed “high”, the adjuvant is “medium” and water and active are both “low”. The reason that the gelling agent is “high” is obvious as the primary function of including this is to establish the correct rheology profile. The reason behind the adjuvant being medium is that sometimes these may be a surfactant and that these can help/hinder with structure formation.
So, this preliminary risk assessment highlights the areas to investigate initially with the aim of establishing more quantitative detail on for example impurities in the active ingredient and ranges for acceptable levels of the active and gelling agent. They also serve as troubleshooting guides if in the future there are any issues with efficacy or rheology.
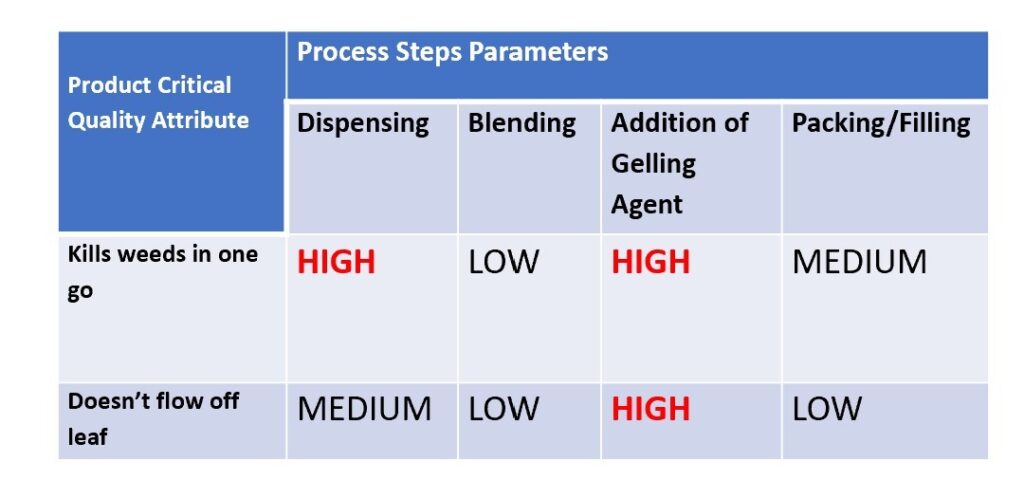
A similar risk assessment would be used to look at process parameters, which in this hypothetical case could be “dispensing”, “Blending”, “Addition of Gelling Agent” and “Packing/Filling”. In our assessment we deemed Dispensing and Addition of Gelling Agent to be high risk relating to “Will work in one go” and “Addition of Gelling Agent” to be high risk for “Doesn’t Flow off Leaf”.
Often risk assessments are presented in a tabular format and these are shown below:
So now we are ready to get to work and in the next post we will look at various approaches to designing experiments.

